Advanced Cell-Free Protein Synthesis Systems Training Course
Advanced Cell-Free Protein Synthesis Systems Training Course is designed to transition participants from foundational knowledge to the mastery of cutting-edge CFPS systems, emphasizing high-throughput screening, synthetic biology integration, and biomanufacturing scalability
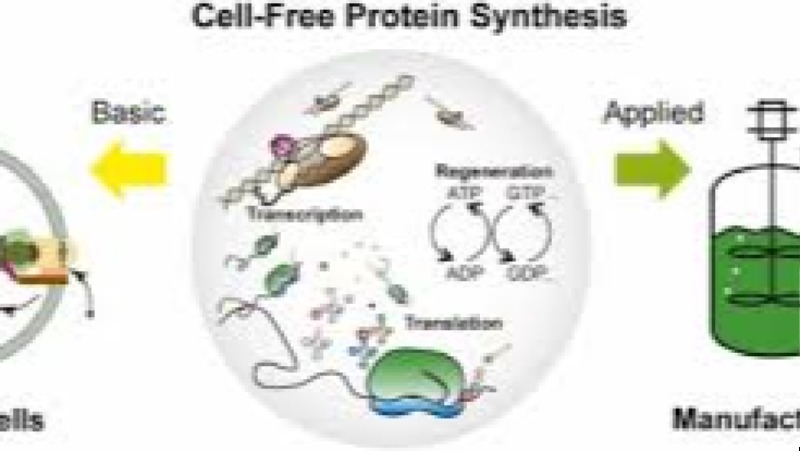
Course Overview
Advanced Cell-Free Protein Synthesis Systems Training Course
Introduction
The landscape of modern biotechnology is being fundamentally transformed by Cell-Free Protein Synthesis (CFPS), an innovative platform that decouples protein production from living cells. Advanced Cell-Free Protein Synthesis Systems Training Course is designed to transition participants from foundational knowledge to the mastery of cutting-edge CFPS systems, emphasizing high-throughput screening, synthetic biology integration, and biomanufacturing scalability. CFPS is no longer a niche research tool; it is now a critical technology for the rapid prototyping of therapeutics, enzymes, and diagnostics, offering unprecedented control over reaction environments and the ability to incorporate non-canonical amino acids. Key benefits include the expression of toxic proteins and membrane proteins, which are often intractable in traditional in vivo systems.
This intensive training delves into the next generation of cell-free technology, including the use of PURE systems, advanced lysate preparation, and the crucial steps for process optimization and lyophilization for enhanced shelf-stability and point-of-care application. Strong keywords like Metabolic Engineering, Genetic Code Expansion, and Microfluidics form the core of the curriculum. Participants will gain practical, hands-on experience in implementing Design of Experiments (DoE) and integrating Machine Learning (ML) for predictive modeling, ensuring they are equipped to drive innovation and lead industrial-scale CFPS projects in the burgeoning biomanufacturing sector.
Course Duration
10 days
Course Objectives
- Master the preparation and Quality Control of diverse High-Yield Lysates
- Design and Engineer optimal Linear DNA Templates and plasmids for maximum CFPS efficiency.
- Implement Genetic Code Expansion techniques for site-specific incorporation of Non-Canonical Amino Acids
- Develop and execute High-Throughput Screening (HTS) assays using miniaturized and Microfluidic CFPS platforms.
- Apply Design of Experiments (DoE) methodologies for systematic Process Optimization and yield maximization.
- Understand the principles and applications of Cell-Free Metabolic Engineering (CFME) for biochemical production.
- Synthesize challenging targets, including Membrane Proteins and complex Multi-Protein Assemblies
- Design and prototype functional Cell-Free Biosensors for Point-of-Care Diagnostics and environmental monitoring.
- Integrate Machine Learning (ML) and Computational Modeling for predictive CFPS workflow optimization.
- Execute Downstream Processing (DSP) and Tagless Purification strategies for high-purity protein isolation.
- Develop protocols for Lyophilization and room-temperature storage of CFPS reaction components and products.
- Conduct comprehensive Cost-Benefit Analysis and strategize for Biomanufacturing scale-up from batch to continuous flow.
- Troubleshoot and De-Risk common CFPS reaction failures, including protease activity and energy depletion.
Target Audience
- Senior Research Scientists in Biopharma and Biotech.
- R&D Managers.
- Process Development Engineers.
- Synthetic Biologists and Bioengineers.
- Postdoctoral Researchers and PhD Students.
- Diagnostics Developers.
- Enzyme Engineers.
- QC/QA Professionals.
Course Modules
Module 1: Foundational CFPS Systems & Components
- Advanced Lysate Preparation.
- The PURE System.
- Optimizing energy regeneration systems and key co-factors for sustained, high-yield reactions.
- Case Study: Comparing the yield and quality of a cytotoxic enzyme produced in E. coli S30 vs. the PURE system.
- Quality control methods for lysate activity, including protein and ribonuclease quantification assays.
Module 2: DNA Template & Vector Engineering
- Template Design
- Advanced Codon Optimization algorithms and their application for rare tRNA depletion in specific CFPS systems.
- Engineering stable, high-expressing RNA templates and cap/tail optimization for eukaryotic CFPS.
- Case Study: Maximizing expression of a complex antibody fragment via tRNA and codon balancing in a mammalian CFPS system.
- Utilization of PCR-free and cloning-free rapid prototyping workflows.
Module 3: Energy and Reaction Optimization
- In-depth analysis of energy sources and their effect on reaction longevity.
- Design of Experiments
- Systematic tuning of Magnesium and Potassium concentrations for specific protein folding requirements.
- Case Study: Using a DoE matrix to optimize four reaction components simultaneously, resulting in a 3-fold increase in vaccine antigen yield.
- Troubleshooting strategies for common inhibitors.
Module 4: High-Throughput Screening & Miniaturization
- Implementing Automated Liquid Handling robotics for multi-plate CFPS library screening.
- Microfluidic CFPS Systems.
- Developing reporter-based assays for rapid activity and yield quantification in HTS.
- Case Study: Screening 1,000+ enzyme variants for enhanced thermostability using a miniaturized CFPS-based library.
- Data analysis and visualization of HTS results using statistical and bioinformatics software.
Module 5: Synthesis of Difficult-to-Express Proteins
- Membrane Protein Expression.
- Strategies for co-expressing and assembling complex Multi-Subunit Complexes.
- Incorporation of specific Chaperones and folding aids into the reaction mix.
- Case Study: Successful production and functional analysis of a G Protein-Coupled Receptor in a modified CFPS system.
- Methods for expressing and stabilizing Toxic Proteins in vitro.
Module 6: Genetic Code Expansion (GCE)
- Fundamentals of GCE.
- Techniques for site-specific incorporation of Non-Canonical Amino Acids for functionalization
- Engineering the CFPS lysate for enhanced ncAAs incorporation efficiency and fidelity.
- Case Study: Creating a fluorescently labeled, site-specifically modified therapeutic protein for enhanced in vivo tracking.
- Quality assessment of GCE efficiency using mass spectrometry and protein labeling assays.
Module 7: Cell-Free Biosensors and Diagnostics
- Design principles for Paper-Based CFPS Diagnostics and their deployment for Point-of-Care (POC) applications.
- Engineering CFPS systems to detect specific nucleic acid targets
- Integrating sophisticated Synthetic Circuits within the cell-free environment.
- Case Study: Developing a lyophilized, room-temperature stable CFPS kit for rapid detection of a viral pathogen in the field.
- Translating biological detection signals into measurable outputs
Module 8: Cell-Free Metabolic Engineering (CFME)
- The use of CFPS as a rapid prototyping platform for de novo and optimized metabolic pathways.
- Pathway Component Optimization.
- Production of fine chemicals, biofuels, and specialized metabolites using cell-free biocatalysis.
- Case Study: Constructing a cell-free pathway for the one-pot synthesis of a high-value precursor molecule.
- Methods for minimizing side-product formation and extending the lifetime of the cell-free pathway.
Module 9: Computational and Machine Learning Integration
- Fundamentals of Kinetic Modeling of CFPS reactions to predict performance.
- Machine Learning (ML).
- Computational tools for DNA Template Design
- Case Study: Utilizing a Bayesian optimization algorithm to iteratively tune buffer conditions, resulting in a novel, high-performance CFPS formulation.
- Bioinformatics pipelines for processing high-volume sequence and activity data from CFPS experiments.
Module 10: Downstream Processing and Purification
- Affinity Tag Purification.
- Implementing Tagless Purification methods for native protein targets.
- Optimizing protein refolding strategies and post-translational modification (PTM) induction in vitro.
- Case Study: Achieving >95% purity of a recombinant growth factor using a two-step, tagless CFPS purification protocol.
- Quantitative protein characterization
Module 11: Biomanufacturing and Scale-Up
- Transitioning from Batch Mode to Continuous-Exchange CFPS and hollow-fiber bioreactors.
- Strategies for maximizing Space-Time-Yield and reaction volume efficiency.
- Developing robust Standard Operating Procedures for industrial-grade CFPS systems.
- Case Study: Scaling up therapeutic peptide production using a 5-liter CECFRS system with continuous feed/waste exchange.
- Regulatory considerations and Good Manufacturing Practices (GMP) for CFPS-produced biotherapeutics.
Module 12: Lyophilization and Stabilization
- Principles of Lyophilization for creating shelf-stable CFPS reagents and diagnostics.
- Selecting and optimizing Excipients and cryoprotectants for maximum component recovery and activity post-rehydration.
- Protocols for packaging and storing lyophilized CFPS components for global distribution.
- Case Study: Demonstrating 1-year stability of a lyophilized CFPS diagnostic kit at ambient temperatures.
- Accelerated stability testing and real-time degradation analysis.
Module 13: Industrial Applications: Vaccines & Therapeutics
- Rapid prototyping and production of Virus-Like Particles for next-generation vaccine development.
- In situ CFPS for the synthesis of Antibody Fragments and other complex biotherapeutics.
- Drug-Toxicity Screening.
- Case Study: Rapid production of an emerging disease VLP vaccine candidate in a CFPS system for Phase I clinical trials.
- Future outlook.
Module 14: Safety and Ethical Considerations
- Best practices for handling and disposing of biological reagents and modified DNA templates.
- Ethical and regulatory implications of using CFPS for gene drive components or synthetic life.
- Intellectual property and patent landscape surrounding advanced CFPS technology and systems.
- Case Study: Discussion on the dual-use concerns of cell-free technology and responsible innovation.
- Laboratory safety protocols tailored for high-throughput, automated CFPS workflows.
Module 15: Advanced Troubleshooting & Process Validation
- Advanced analytical methods for diagnosing CFPS failures.
- Techniques for removing or neutralizing nucleases, proteases, and translation inhibitors in crude lysates.
- Implementing Process Analytical Technology (PAT) for real-time monitoring of CFPS reaction kinetics.
- Case Study: Troubleshooting a low-yield CFPS reaction by identifying a critical cofactor depletion point using in-line sensor technology.
- Validation and documentation procedures for reproducing high-quality CFPS results across different labs.
Training Methodology
This course employs a participatory and hands-on approach to ensure practical learning, including:
- Interactive lectures and presentations.
- Group discussions and brainstorming sessions.
- Hands-on exercises using real-world datasets.
- Role-playing and scenario-based simulations.
- Analysis of case studies to bridge theory and practice.
- Peer-to-peer learning and networking.
- Expert-led Q&A sessions.
- Continuous feedback and personalized guidance.
Register as a group from 3 participants for a Discount
Send us an email: info@datastatresearch.org or call +254724527104
Certification
Upon successful completion of this training, participants will be issued with a globally- recognized certificate.
Tailor-Made Course
We also offer tailor-made courses based on your needs.
Key Notes
a. The participant must be conversant with English.
b. Upon completion of training the participant will be issued with an Authorized Training Certificate
c. Course duration is flexible and the contents can be modified to fit any number of days.
d. The course fee includes facilitation training materials, 2 coffee breaks, buffet lunch and A Certificate upon successful completion of Training.
e. One-year post-training support Consultation and Coaching provided after the course.
f. Payment should be done at least a week before commence of the training, to DATASTAT CONSULTANCY LTD account, as indicated in the invoice so as to enable us prepare better for you.