Antibody-Drug Conjugates (ADCs) Design and Synthesis Training Course
Antibody-Drug Conjugates (ADCs) Design and Synthesis Training Course is designed to equip R&D scientists, medicinal chemists, and drug developers with the specialized, cutting-edge knowledge required to design and synthesize high-quality, homogeneous ADCs.
Skills Covered
Course Overview
Antibody-Drug Conjugates (ADCs) Design and Synthesis Training Course
Introduction
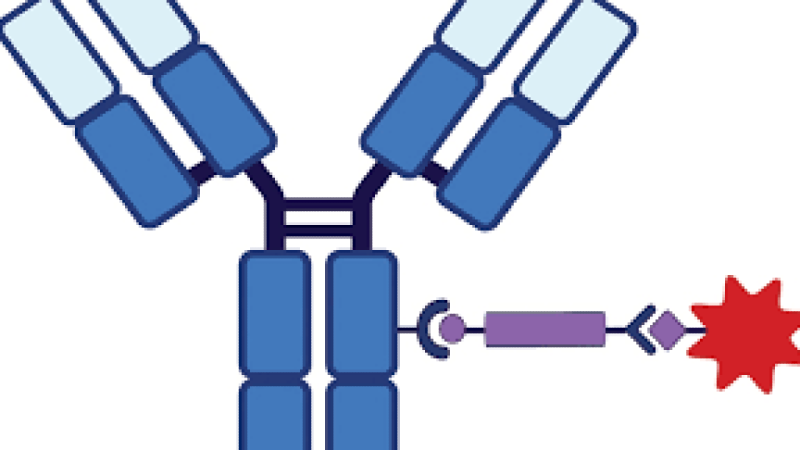
Antibody-Drug Conjugates (ADCs) represent a paradigm shift in precision medicine, marrying the exquisite target specificity of monoclonal antibodies with the potent cytotoxicity of small-molecule drugs. This innovative class of biopharmaceuticals is at the forefront of oncology and infectious disease research, offering a high-impact therapeutic index and minimizing systemic toxicity compared to traditional chemotherapy. The successful development of next-generation ADCs, however, relies on mastering the complexities of the ADC triad: the antibody, the stable yet cleavable linker chemistry, and the cytotoxic payload. A deep and integrated understanding of bioconjugation strategies, pharmacokinetics (PK), and the latest regulatory and manufacturing challenges is essential to unlock their full clinical potential and drive forward the future of targeted therapy.
Antibody-Drug Conjugates (ADCs) Design and Synthesis Training Course is designed to equip R&D scientists, medicinal chemists, and drug developers with the specialized, cutting-edge knowledge required to design and synthesize high-quality, homogeneous ADCs. Focusing on practical applications and data-driven design, the curriculum will delve into advanced topics such as site-specific conjugation, novel payload modalities, and the role of Artificial Intelligence (AI) in optimizing the therapeutic window. Participants will gain the critical skills to troubleshoot common development hurdles, optimize the Drug-to-Antibody Ratio (DAR), and navigate the intricate landscape of preclinical and clinical translation, accelerating the delivery of safer and more efficacious biologics to patients.
Course Duration
10 days
Course Objectives
- Master the fundamental principles of the ADC Triad and their synergistic impact on Therapeutic Index.
- Evaluate various Target Antigen selection criteria for optimal tumor specificity and internalization kinetics.
- Design and select appropriate next-generation cytotoxic payloads, including DNA-damaging agents and tubulin inhibitors, based on potency and mechanism of action.
- Analyze and compare modern Linker Chemistry designs, focusing on stability, release mechanisms, and serum half-life.
- Implement and optimize Site-Specific Bioconjugation techniques for homogeneous ADC production and defined DAR.
- Interpret and leverage Pharmacokinetics (PK) and Pharmacodynamics (PD) data to predict in vivo efficacy and safety profiles.
- Apply advanced Analytical Characterization methods for quality control and Critical Quality Attribute (CQA) assessment.
- Understand the mechanisms of ADC Resistance and develop rational strategies for combination therapy and overcoming acquired resistance.
- Explore the emerging use of AI/Machine Learning in ADC Drug Discovery for optimization of molecular design.
- Discuss the regulatory and Chemistry, Manufacturing, and Controls requirements for transitioning an ADC candidate from preclinical to clinical trials.
- Evaluate the potential of Non-Oncology ADCs and other Targeted Biologics applications.
- Formulate strategies for managing and mitigating common Dose-Limiting Toxicities (DLTs) associated with payload and off-target effects.
- Generate a viable Next-Generation ADC Design Concept with an optimized DAR and enhanced payload stability.
Target Audience
- Medicinal Chemists and Synthetic Chemists
- Biopharmaceutical R&D Scientists
- Process Development and CMC Engineers
- Molecular and Cell Biologists
- Toxicologists and Preclinical Safety Specialists
- Biologics Formulation Scientists
- Project Managers overseeing Targeted Biologics pipelines
- Regulatory Affairs Professionals specializing in biologics
Course Modules
Module 1: ADC Fundamentals and The Triad's Role
- Introduction to ADCs.
- Antibody, Linker, and Payload, and their synergistic function.
- Mechanism of Action.
- Defining the Therapeutic Index and Window in ADC development.
- Case Study: Analysis of the first-generation ADC, Mylotarg, to understand early design flaws and subsequent clinical failure/re-approval.
Module 2: Monoclonal Antibody (mAb) Selection and Engineering
- Criteria for ideal Target Antigen selection
- Role of mAb format, isotype, and effector function in ADC design.
- Methods for Antibody Engineering.
- Impact of Fc-mediated effector functions on ADC efficacy and toxicity.
- Case Study: Comparing the anti-HER2 antibodies used in Kadcyla and Enhertu to highlight the importance of internalization and payload delivery.
Module 3: Cytotoxic Payload Design and Mechanism
- Classes of cytotoxic payloads
- Required payload properties.
- Impact of payload lipophilicity on ADC aggregation and non-specific toxicity.
- Strategies for maximizing Bystander Effect and Target Cell Killing.
- Case Study: Deep dive into the mechanism of PBD-based payloads and the challenges of managing their extreme potency and toxicity profile.
Module 4: Linker Chemistry and Cleavage Strategies
- Cleavable Linkers
- Non-Cleavable Linkers.
- The importance of Linker Stability in circulation to prevent premature drug release and systemic toxicity.
- Balancing hydrophilicity and hydrophobicity in linker design.
- Case Study: Contrasting the valine-citrulline cleavable linker with non-cleavable linkers to understand clinical stability and payload release kinetics.
Module 5: Random Conjugation Techniques
- Conventional conjugation via lysine and cysteine residues
- Chemistry and limitations of Cysteine-based Thiol Conjugation.
- Impact of Lysine Conjugation on heterogeneity and defined DAR.
- The challenge of product heterogeneity and its impact on PK, efficacy, and manufacturability.
- Case Study: Examining the conjugation method for Adcetri and the complexities of manufacturing a heterogenous product mixture.
Module 6: Advanced Site-Specific Conjugation (SSC)
- Introduction to next-generation Site-Specific Conjugation for homogeneous ADCs.
- Enzymatic Conjugation.
- Genetic Engineering.
- Aldehyde tagging, formylglycine-generating enzyme conjugation.
- Case Study: Review of the THIOMAB technology and its role in achieving a near-perfect DAR of 2.0 or 4.0 for improved therapeutic profiles.
Module 7: Analytical Characterization and Quality Control (QC)
- Quantifying the Drug-to-Antibody Ratio using Hydrophobic Interaction Chromatography
- Advanced characterization using Mass Spectrometry and intact mass analysis.
- Assessing aggregation, fragmentation, and charge variants
- Techniques for determining ADC stability and impurity profiles.
- Case Study: Troubleshooting an unexpected high-molecular-weight species in an ADC batch and using advanced MS techniques to identify the aggregate structure.
Module 8: Pharmacokinetics (PK) and Bioanalysis
- Understanding the In Vivo fate of the ADC, released payload, and catabolites.
- PK modeling of the tri-component system
- Bioanalytical assays for measuring ADC concentration in plasma and tissue
- Role of target expression and binding affinity in distribution and clearance.
- Case Study: Modeling the difference in tumor exposure and systemic clearance between an ADC with a stable linker versus one with premature payload release.
Module 9: Preclinical Efficacy and Toxicology
- Designing and interpreting in vitro cytotoxicity, internalization, and bystander effect assays.
- Selection of appropriate tumor xenograft and patient-derived xenograft models.
- Non-GLP and GLP toxicology studies to assess off-target and payload-related toxicity.
- Identifying and managing key Dose-Limiting Toxicities
- Case Study: Analysis of preclinical toxicology data for an ADC candidate that was successfully advanced by identifying a correctable off-target toxicity mechanism.
Module 10: Process Development and CMC
- Overview of Good Manufacturing Practice for ADC production.
- Upstream and Downstream processing challenges for mAb production and purification.
- Optimization of the Bioconjugation Process for large-scale, reproducible synthesis.
- Filtration, lyophilization, and final product formulation and stability.
- Case Study: Developing a robust and scalable conjugation and purification process that meets regulatory standards and minimizes solvent use for environmental impact.
Module 11: Regulatory and Quality Assurance
- Global regulatory requirements for ADCs
- Process validation, specification setting, and comparability studies.
- Quality control strategies for controlling DAR heterogeneity and free drug levels.
- Importance of Drug-Device Combination Product considerations for administration.
- Case Study: Navigating a regulatory agency's request for additional data on a specific Critical Quality Attribute (CQA) related to product stability.
Module 12: ADC Resistance and Combination Strategies
- Mechanisms of ADC Resistance.
- Strategies to overcome resistance, including alternative targets or novel payloads.
- Rational design of Combination Therapies
- Exploring bispecific antibodies and other innovative formats.
- Case Study: Designing a clinical trial strategy for an ADC in combination with a checkpoint inhibitor to achieve synergistic anti-tumor activity.
Module 13: Emerging ADCs and Non-Oncology Applications
- Next-Generation ADCs
- The potential of ADCs beyond cancer.
- Exploring the role of new conjugation platforms like hydrogel-based linkers.
- Overview of Radioconjugates and their relationship to ADCs.
- Case Study: Hypothetical development plan for a non-oncology ADC targeting a pathogenic antigen in a severe infectious disease.
Module 14: Computational Design and AI in ADCs
- Application of Computational Chemistry for payload design and optimization.
- Using Molecular Modeling to predict linker stability and cleavage kinetics.
- Leveraging Artificial Intelligence and Machine Learning for antigen-antibody pairing.
- In silico prediction of PK/PD and early toxicity endpoints.
- Case Study: Using an ML model trained on historical ADC data to rank potential payload-linker combinations for a new antibody target.
Module 15: Future Outlook and Commercialization
- Analysis of the current ADC Clinical Pipeline and new approvals.
- Manufacturing scaling and cost-of-goods challenges in commercialization.
- Strategies for intellectual property and patent protection in the crowded ADC space.
- Future directions: Oral ADCs and localized delivery systems.
- Case Study: Forecasting the commercial trajectory and market penetration of a recently approved ADC versus its competitors.
Training Methodology
The course employs a highly interactive, blended learning approach centered on real-world pharmaceutical development.
- Expert-Led Lectures.
- Hands-on Workshops/Simulations.
- In-Depth Case Studies.
- Group Discussions and Peer Review.
- Q&A/Consultation.
Register as a group from 3 participants for a Discount
Send us an email: info@datastatresearch.org or call +254724527104
Certification
Upon successful completion of this training, participants will be issued with a globally- recognized certificate.
Tailor-Made Course
We also offer tailor-made courses based on your needs.
Key Notes
a. The participant must be conversant with English.
b. Upon completion of training the participant will be issued with an Authorized Training Certificate
c. Course duration is flexible and the contents can be modified to fit any number of days.
d. The course fee includes facilitation training materials, 2 coffee breaks, buffet lunch and A Certificate upon successful completion of Training.
e. One-year post-training support Consultation and Coaching provided after the course.
f. Payment should be done at least a week before commence of the training, to DATASTAT CONSULTANCY LTD account, as indicated in the invoice so as to enable us prepare better for you.