Advanced Process Validation & Cleaning Validation Training Course
Advanced Process Validation & Cleaning Validation Training Course is meticulously designed to move beyond foundational GMP and GxP concepts, focusing on the modern, Science and Risk-Based Approach mandated by global regulatory bodies.
Skills Covered
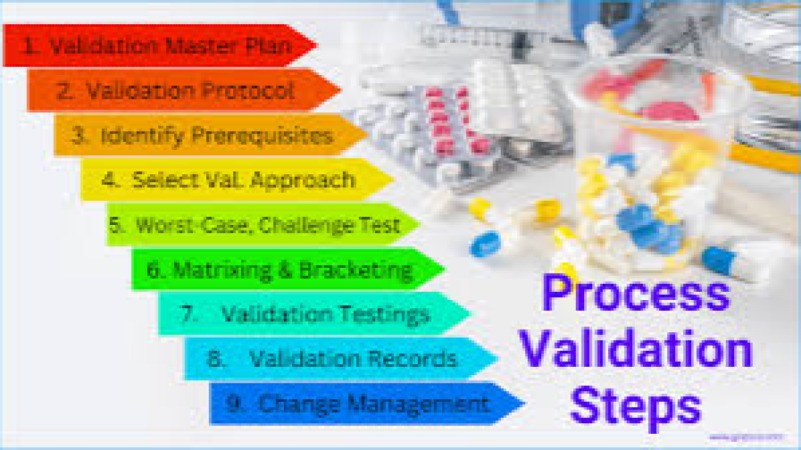
Course Overview
Advanced Process Validation & Cleaning Validation Training Course
Introduction
In the highly regulated pharmaceutical and biotech sectors, ensuring product quality and patient safety is non-negotiable. Advanced Process Validation & Cleaning Validation Training Course is meticulously designed to move beyond foundational GMP and GxP concepts, focusing on the modern, Science and Risk-Based Approach mandated by global regulatory bodies. The core challenge for industry professionals is translating complex Quality by Design (QbD) principles into a robust, three-stage validation lifecycle: Process Design, Process Performance Qualification (PPQ), and Continued Process Verification (CPV). Mastering these stages is essential to achieve a state of control and defend manufacturing processes during increasingly stringent regulatory inspections.
This training provides a deep dive into the interconnected disciplines of advanced Process Validation and Cleaning Validation. Participants will gain strategic knowledge in critical areas, including the application of Quality Risk Management (QRM) to define Critical Process Parameters (CPPs) and Critical Quality Attributes (CQAs). A significant focus is placed on the technical mastery of Cleaning Validation, particularly in multi-product facilities, by establishing scientifically justified limits based on Health-Based Exposure Limits (HBELs), such as PDE/ADE. By integrating advanced statistical tools and ensuring Data Integrity throughout the entire product lifecycle, attendees will be equipped to develop, execute, and maintain validation programs that drive operational excellence and achieve sustainable compliance.
Course Duration
10 days
Course Objectives
Upon completion, participants will be able to:
- Apply the FDA Process Validation Lifecycle and ICH Q8, Q9, Q10 frameworks.
- Implement a robust Quality Risk Management (QRM) process to identify and control Critical Process Parameters (CPPs).
- Design and execute Process Performance Qualification (PPQ) protocols that leverage advanced Statistical Process Control (SPC) tools.
- Establish and maintain a Continued Process Verification (CPV) program using Trend Analysis and Process Capability Index (CpK).
- Develop a Cross-Contamination Control Strategy compliant with EMA and PIC/S expectations.
- Calculate scientifically justifiable Health-Based Exposure Limits (HBELs), including PDE and ADE.
- Determine and justify Maximum Allowable Carryover (MACO) and Surface Area Limit (SAL) for cleaning residues.
- Design Worst-Case Scenario protocols for multi-product equipment and complex residue matrices.
- Validate modern cleaning technologies and complex manual cleaning procedures.
- Select, validate, and justify advanced Analytical Methods for residue detection, including recovery studies.
- Implement Data Integrity (ALCOA+) principles across all validation documentation and electronic systems.
- Manage Dirty and Clean Hold Times using statistically supported kinetics and studies.
- Successfully defend validation strategies during Pre-Approval Inspections (PAI) and Regulatory Audits.
Target Audience
- Validation Engineers/Specialists
- Quality Assurance (QA) Managers and Specialists
- Manufacturing/Production Managers and Supervisors
- Cleaning/Sanitization Specialists and Technicians
- Quality Control (QC) Laboratory Analysts
- Regulatory Affairs Professionals
- Process Development and R&D Scientists
- Engineering and Maintenance Staff
Course Modules
Module 1: PV Regulatory Landscape & Science-Based Approach
- FDA Process Validation Guidance and EMA Annex 15.
- Translating ICH Q8 (QbD) principles into the validation design space.
- Integrating the Quality Risk Management process into PV planning.
- Defining and linking CQAs to CPPs and control strategy.
- Case Study: Analysis of a recent FDA Warning Letter citing failure to maintain a validated state (Stage 3 CPV).
Module 2: Process Design & Process Understanding (PV Stage 1)
- Utilizing Design of Experiments (DoE) for robust process optimization.
- Identifying and characterizing sources of process Variability and Noise.
- Development of a comprehensive Control Strategy for all process steps.
- Scale-Up considerations and validation prior to commercialization.
- Case Study: Applying DoE to optimize a critical mixing or coating CPP to establish a proven acceptable range.
Module 3: Process Performance Qualification (PPQ) (PV Stage 2)
- Developing statistically sound PPQ protocols and robust sampling plans.
- Justifying the number of required consecutive successful batches.
- Setting appropriate, scientifically justified Acceptance Criteria.
- Managing and documenting PPQ Deviations and atypical events.
- Case Study: Reviewing a PPQ failure, determining the root cause, and developing a corrective PPQ strategy.
Module 4: Continued Process Verification (CPV) (PV Stage 3)
- Designing and implementing a comprehensive CPV Plan for ongoing control.
- Selection and use of appropriate Statistical Tools
- Performing ongoing Data Trending and periodic data review.
- Annual Product Review (APR) integration with CPV data and conclusions.
- Case Study: Utilizing a control chart to identify a process drift before it resulted in a batch rejection
Module 5: Facility, Equipment, and Utility Qualification Link to PV
- The essential connection between Design Qualification (DQ) and PV.
- Advanced concepts in IQ/OQ/PQ and how they impact CPPs.
- Critical Utilities Validation and their effect on product CQA.
- Managing Legacy Equipment validation and gap analysis.
- Case Study: Documenting the validation of a new high-shear mixer (PQ) and its successful integration into a PPQ study.
Module 6: CV Regulatory Mandate & Cross-Contamination Control
- Detailed review of HBEL and their regulatory foundation.
- Developing a facility-wide Cross-Contamination Control Strategy.
- Regulatory expectations for Dedicated and Multi-Product Equipment.
- Compliance with EMA and PIC/S specific guidance on cleaning.
- Case Study: Analyzing a company's response to an audit finding on inadequate cross-contamination risk management in a shared facility.
Module 7: Calculating Health-Based Exposure Limits (HBEL)
- Step-by-step calculation of Permitted Daily Exposure (PDE) based on toxicological data.
- Deriving the Acceptable Daily Exposure (ADE) for complex materials.
- Calculating the final Maximum Allowable Carryover (MACO) limit.
- Converting MACO to site-specific Surface Area Limits (SAL) and rinse limits.
- Case Study: Calculating PDE/ADE and MACO for a highly potent API in a campaign-based manufacturing train.
Module 8: Cleaning Process Development & Worst-Case Logic
- Optimizing Cleaning Agents and determining effective Cleaning Mechanisms.
- Defining and validating Critical Cleaning Parameters
- Selecting the scientific Worst-Case Product and Worst-Case Equipment Train.
- Bracketing and Grouping strategies for multiple products and equipment.
- Case Study: Justifying the worst-case product for a cleaning validation study involving a low-solubility, high-potency compound.
Module 9: Cleaning Hold Times and Revalidation
- Designing and executing studies for Dirty Hold Time (DHT) validation.
- Establishing and justifying Clean Hold Time (CHT) limits.
- Managing revalidation triggers.
- Risk-based approach to periodic re-evaluation and re-qualification.
- Case Study: Designing a stability-based DHT study for a product prone to 'caking' on stainless steel surfaces.
Module 10: Validating Analytical Methods for Residue Testing
- Validation requirements for analytical methods.
- Advanced principles of Recovery Study design and acceptance criteria.
- Selecting the right method
- Managing non-recoverable surfaces and Statistical Analysis of recovery data.
- Case Study: A hands-on exercise on determining the appropriate spike level and calculating the percentage recovery for a swab sample.
Module 11: Sampling Strategies & Techniques
- Developing scientifically justified Swab Sampling locations and techniques.
- Designing robust Rinse Sampling protocols for CIP/COP systems.
- Establishing Visual Inspection as a justified criterion.
- Identifying and justifying Most Difficult to Clean (MDC) locations.
- Case Study: Drafting a comprehensive sampling plan for a complex reactor vessel with internal baffles and spray balls.
Module 12: Automated Cleaning (CIP/COP) Validation
- Qualification of Clean-in-Place (CIP) and Clean-Out-of-Place (COP) systems.
- Validation of automated processes.
- Spray Ball Coverage Test validation and documentation.
- Integrating Automation (CSV) into the CIP validation protocol.
- Case Study: Analyzing a CIP program's validation data to confirm complete coverage and consistent delivery of the cleaning solution.
Module 13: Data Integrity (DI) in Validation and Records
- Implementing ALCOA+ principles for all validation records.
- Ensuring Audit Trail compliance for electronic validation data.
- Validation of Digital Validation Management Systems
- Regulatory expectations for Electronic Signatures and Raw Data.
- Case Study: Reviewing a fictional DI violation in a cleaning validation report and defining the necessary CAPA.
Module 14: Validation Master Planning & Documentation
- Developing a compliant Cleaning Validation Master Plan and a VMP for PV.
- Essential content for validation Protocols and Final Reports.
- Writing clear, concise, and defensible Cleaning SOPs.
- Effective management of Change Control and its impact on validation status.
- Case Study: A workshop on structuring and reviewing a Cleaning Validation Protocol to ensure it is audit-ready.
Module 15: Regulatory Inspection Readiness & Defense
- Preparing validation documentation for a Pre-Approval Inspection (PAI).
- Mock Audit preparation focused on validation and cleaning documentation.
- Strategies for effectively Defending validation reports and rationales to inspectors.
- Responding to and addressing Regulatory Observations.
- Case Study: Role-playing an inspector/auditee scenario focused on justifying HBEL calculations or a CHT study.
Training Methodology
The course employs an intensive, application-focused methodology to ensure immediate real-world utility:
- Expert-Led Lectures.
- Interactive Workshops.
- Real-World Case Studies.
- Protocol Development Exercises
- Q&A/Discussion Forums.
Register as a group from 3 participants for a Discount
Send us an email: info@datastatresearch.org or call +254724527104
Certification
Upon successful completion of this training, participants will be issued with a globally- recognized certificate.
Tailor-Made Course
We also offer tailor-made courses based on your needs.
Key Notes
a. The participant must be conversant with English.
b. Upon completion of training the participant will be issued with an Authorized Training Certificate
c. Course duration is flexible and the contents can be modified to fit any number of days.
d. The course fee includes facilitation training materials, 2 coffee breaks, buffet lunch and A Certificate upon successful completion of Training.
e. One-year post-training support Consultation and Coaching provided after the course.
f. Payment should be done at least a week before commence of the training, to DATASTAT CONSULTANCY LTD account, as indicated in the invoice so as to enable us prepare better for you.