Gmp Courses
Explore instructor-led and hybrid programs aligned to practical Gmp use cases across industries.
Available Gmp Courses
Showing 1-8 of 8 courses
Biotechnology and Pharmaceutical Development
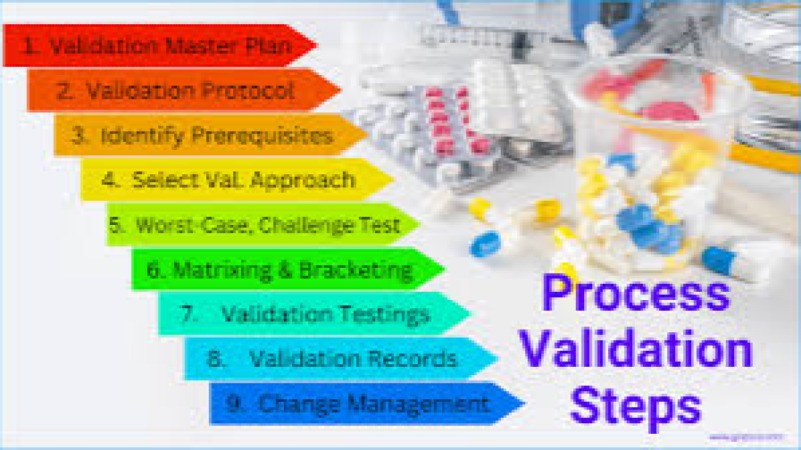
Advanced Process Validation & Cleaning Validation Training Course is meticulously designed to move beyond foundational GMP and GxP concepts, focusing on the modern, Science and Risk-Based Approach mandated by global regulatory bodies.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Process Validation & Cleaning Validation Training Course is meticulously designed to move beyond foundational GMP and GxP concepts, focusing on the modern, Science and Risk-Based Approach mandated by global regulatory bodies.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Advanced Quality Assurance in the Blood and Tissue Industry Training Course is engineered for QA and Regulatory Affairs leaders seeking to solidify their expertise in the unique challenges of Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) and blood product manufacturing.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Quality Assurance in the Blood and Tissue Industry Training Course is engineered for QA and Regulatory Affairs leaders seeking to solidify their expertise in the unique challenges of Human Cell, Tissue, and Cellular and Tissue-Based Product (HCT/P) and blood product manufacturing.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Advanced Validation of Aseptic Processes Training Course empowers quality and manufacturing professionals to lead comprehensive Aseptic Validation programs, transforming compliance from a reactive measure into a proactive Operational Excellence driver.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Advanced Validation of Aseptic Processes Training Course empowers quality and manufacturing professionals to lead comprehensive Aseptic Validation programs, transforming compliance from a reactive measure into a proactive Operational Excellence driver.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Bioprocess Equipment Qualification (IQ, OQ, PQ) Training Course is engineered to provide a deep, practical mastery of Bioprocess Equipment Qualification (EQ) specifically, the three critical pillars of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Bioprocess Equipment Qualification (IQ, OQ, PQ) Training Course is engineered to provide a deep, practical mastery of Bioprocess Equipment Qualification (EQ) specifically, the three critical pillars of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Food Safety and Biotech Applications (ISO 22000) Training Course provides a comprehensive understanding of the ISO 22000 standard, the international benchmark for food safety management systems (FSMS).
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Food Safety and Biotech Applications (ISO 22000) Training Course provides a comprehensive understanding of the ISO 22000 standard, the international benchmark for food safety management systems (FSMS).
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Food processing and Technology
Infant Formula Processing and Sterilization Training Course is designed to equip participants with specialized knowledge and hands-on expertise in modern infant nutrition production.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Infant Formula Processing and Sterilization Training Course is designed to equip participants with specialized knowledge and hands-on expertise in modern infant nutrition production.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
Sterile Product Manufacturing and Aseptic Processing Training Course is designed to equip professionals with the essential skills to manage aseptic processes and comply with strict industry standards to avoid contamination and ensure the integrity of sterile drugs.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
Sterile Product Manufacturing and Aseptic Processing Training Course is designed to equip professionals with the essential skills to manage aseptic processes and comply with strict industry standards to avoid contamination and ensure the integrity of sterile drugs.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore

Biotechnology and Pharmaceutical Development
The Advanced Viral Vector Manufacturing for Gene Therapy Training Course is designed to bridge the gap between process development and commercial biomanufacturing.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore
The Advanced Viral Vector Manufacturing for Gene Therapy Training Course is designed to bridge the gap between process development and commercial biomanufacturing.
10 days
Accra, Addis Ababa, Bangkok, Cape Town, Dubai, Istanbul, Kigali, Nairobi, Singapore